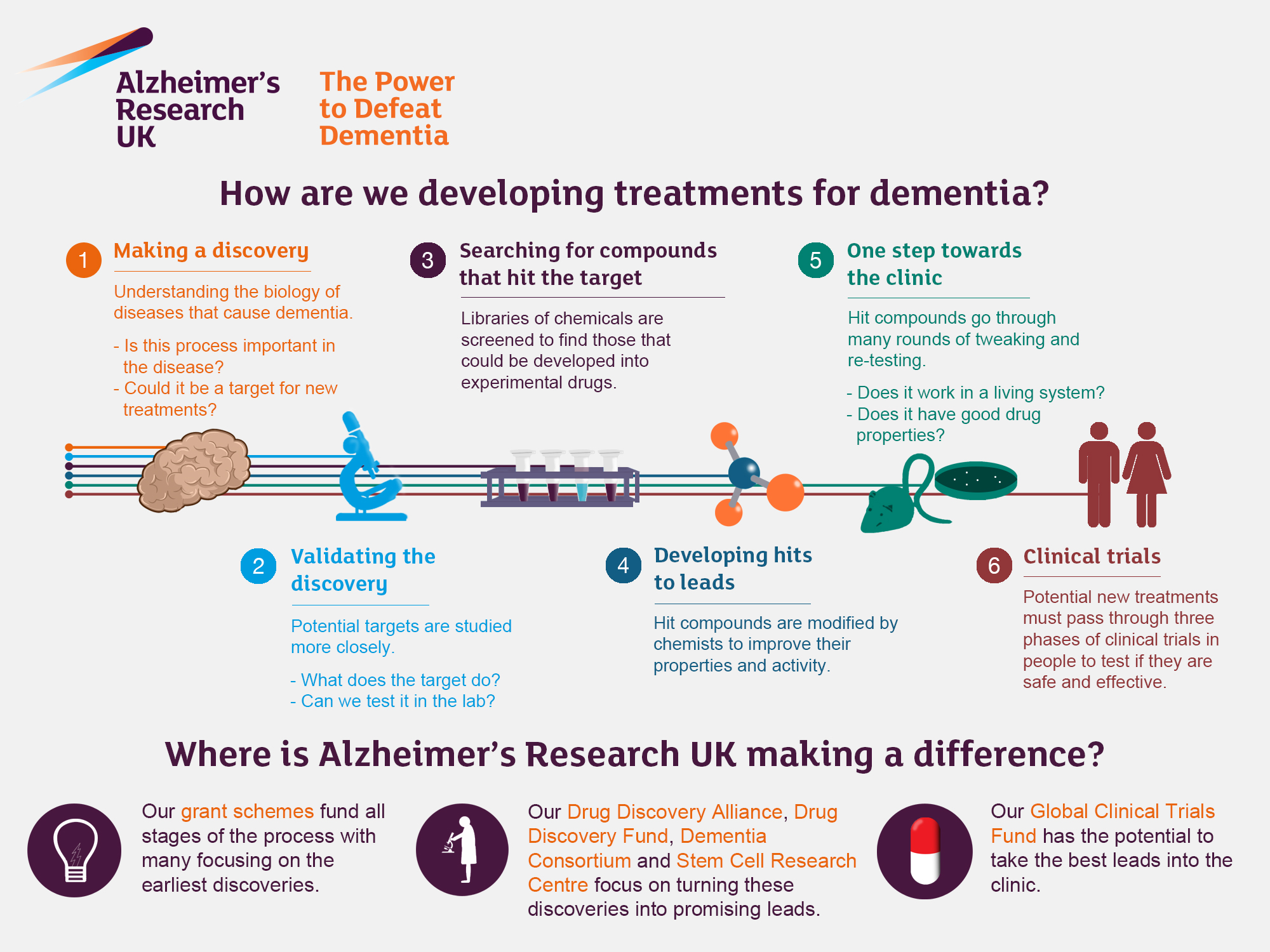
Alzheimer’s research has become a focal point in the fight against one of the most debilitating neurodegenerative diseases of our time. Pioneering work by neuroscientist Beth Stevens has illuminated the crucial role of microglial cells, the brain’s immune system that helps maintain neural health. These cells are responsible for pruning synapses, yet their dysfunction can lead to severe cognitive decline typical of Alzheimer’s disease. As the population ages, understanding these biological mechanisms is essential not only for developing effective Alzheimer’s treatment but also for detecting the disease earlier through innovative biomarkers. With approximately 7 million Americans affected, advancing Alzheimer’s research is critical for improving lives and potentially curbing the anticipated surge in healthcare costs associated with this condition by 2050.
Investigating Alzheimer’s disease requires an in-depth understanding of the complex interplay within our brain’s immune system—a topic that has gained much traction in recent years. Research efforts in this field, led by experts like Beth Stevens, have spotlighted the function of microglial cells in maintaining neural health and their detrimental role in neurodegeneration when mismanaged. These immune cells are integral in the regulation of synaptic pruning, a process that, while essential during brain development, can contribute to pathologies like Alzheimer’s when disrupted. Thus, the broader implications of such studies extend beyond mere understanding; they pave the way for novel strategies in Alzheimer’s treatment and the early detection of cognitive impairments. As the conversation around Alzheimer’s research continues to evolve, so too does the hope for effective interventions in the face of an impending public health crisis.
Understanding Microglial Cells in Alzheimer’s Research
Microglial cells play a critical role in the brain’s immune system, acting as sentinels that monitor neuronal health. Recent research, particularly by pioneering neuroscientist Beth Stevens, has shed light on their dual role in both protecting and potentially harming neural pathways. Through a process known as synaptic pruning, these cells remove dead or damaged neurons, contributing crucially to brain health. However, when this process goes awry, as seen in Alzheimer’s disease, it can lead to neurodegeneration and an exacerbation of cognitive decline. As Stevens illustrates in her findings, abnormal microglial activity can significantly influence the onset and progression of various neurodegenerative diseases, including Alzheimer’s.
The significance of understanding microglial dynamics cannot be overstated, especially with the alarming rise in Alzheimer’s diagnoses projected over the next decades. As researchers continue to unravel the complexities of microglial behavior, they are not only identifying markers for early detection of Alzheimer’s but are also paving the way for innovative treatments. This includes developing therapies aimed at restoring normal microglial function, which may ultimately halt or even reverse neurodegenerative processes. Stevens’ dedication to this vital area of research exemplifies a transformative approach to understanding the brain’s immune response and its implications for diseases such as Alzheimer’s.
New Perspectives on Neurodegenerative Disease Treatment
The landscape of Alzheimer’s treatment is evolving, with groundbreaking discoveries reshaping our understanding of how to combat neurodegenerative diseases. As Beth Stevens emphasizes, the insights gained from studying microglial cells are providing new avenues for therapeutic approaches. By targeting the mechanisms of synaptic pruning and the immune responses in the brain, researchers aim to develop more effective interventions that not only treat symptoms but address underlying causes. The integration of these findings into clinical practice could change the trajectory for millions affected by Alzheimer’s and other similar disorders.
Moreover, with the increasing prevalence of neurodegenerative diseases globally, there’s an urgent need for novel solutions. Traditional approaches have focused primarily on symptomatic relief, but the recognition of the role of microglial cells in disease progression signals a paradigm shift in treatment strategies. Stevens’ research, funded significantly by the National Institutes of Health, underscores the importance of continued investment in fundamental science to foster the development of these innovative therapies. As our understanding deepens, we can hope for breakthrough treatments that will not only improve quality of life but may also substantially reduce healthcare costs associated with Alzheimer’s care.
The Importance of Basic Science in Alzheimer’s Research
Beth Stevens’ journey in Alzheimer’s research highlights the crucial role of basic science in uncovering the mysteries of neurodegeneration. Initially perceived as a niche study of mouse visual systems, her work with microglial cells has led to groundbreaking insights relevant to human health. This illustrates a common pathway in scientific inquiry where fundamental research paves the way for practical applications. By exploring the immune functions of microglia, Stevens has revealed how foundational studies can inform larger questions surrounding neurodegenerative diseases, demonstrating that what may seem disconnected can eventually lead to transformative treatments.
Moreover, Stevens’ achievements serve as an inspiring reminder of the long-term impacts of curiosity-driven research. Gaining Federal support has been pivotal in allowing researchers like her to pursue innovative ideas that challenge the status quo. Each discovery contributes to a broader understanding of diseases like Alzheimer’s, ultimately guiding new strategies for intervention. As scientists address the complexities of the brain and its immune system, the intricate dance between fundamental research and practical outcomes illustrates how vital it is to support and prioritize basic science in the quest to combat Alzheimer’s Disease and other neurodegenerative afflictions.
Innovative Biomarkers and Early Detection of Alzheimer’s
Early detection of Alzheimer’s disease is crucial for effective intervention, and research into microglial cells is leading to the identification of innovative biomarkers. Beth Stevens’ work underscores how understanding immune responses in the brain can help create tests that detect the onset of Alzheimer’s before significant cognitive decline occurs. By pinpointing specific microglial activity associated with neurodegeneration, scientists aim to develop blood tests or imaging techniques that provide earlier diagnoses, enabling patients and families to seek treatment options sooner.
The implications of such advancements are enormous, particularly as we face an aging population increasingly at risk for Alzheimer’s. With millions expected to be diagnosed in the coming years, the ability to diagnose the disease early could transform treatment pathways and improve outcomes. Stevens’ findings contribute to this goal by exploring how microglial dysfunction correlates with early signs of Alzheimer’s. Thus, investing in research that explores biomarkers linked to brain immune responses will be critical in not only detecting Alzheimer’s but also in potentially slowing its progression through timely therapeutic interventions.
Advancements in Alzheimer’s Treatment Strategies
Research into Alzheimer’s treatment strategies has gained momentum as scientists uncover the roles of microglial cells within the brain’s immune system. Beth Stevens’ groundbreaking investigations have provided insights into how these cells contribute to neuronal health and how their malfunction can lead to neurodegenerative diseases. Her focus on the relationship between microglial behavior and Alzheimer’s pathology showcases the potential for developing targeted therapies that can enhance brain health by restoring proper synaptic pruning processes. This innovative line of inquiry holds promise for creating treatments that address the root causes of Alzheimer’s.
Furthermore, the advancements in understanding the molecular mechanisms that govern microglial activity could lead to the creation of drugs that can either boost the protective functions of these immune cells or inhibit their harmful activities. This dual approach may provide a more comprehensive strategy in managing Alzheimer’s disease. As researchers like Stevens continue to explore this vital area of neuroscience, the hope is to transform these findings into effective treatments that can drastically improve the lives of individuals affected by Alzheimer’s, thereby addressing both the symptoms and underlying disease processes.
Impact of Healthcare Costs Due to Alzheimer’s Disease
The economic implications of Alzheimer’s disease are staggering, with projections indicating a potential increase in care costs from $360 million to $1 trillion by 2050. This alarming forecast highlights the urgent need for effective treatments and preventive strategies. Research like that conducted by Beth Stevens not only illuminates the pathophysiology of Alzheimer’s but also speaks to a broader need for addressing healthcare expenditure related to neurodegenerative diseases. By developing therapies that mitigate the impact of the disease on patients and healthcare systems, the financial burden associated with Alzheimer’s can be alleviated.
Understanding the link between Alzheimer’s research and economic outcomes is critical for policymakers and healthcare leaders. By investing in foundational research focused on microglial cells and their role in neurodegeneration, we can potentially reduce the burden of care for the aging population affected by Alzheimer’s disease. Implementing effective treatments derived from such research not only improves patient quality of life but also represents a proactive approach to managing healthcare costs associated with increasing Alzheimer’s prevalence.
Role of Federal Funding in Alzheimer’s Research
Federal funding has played a pivotal role in propelling advancements in Alzheimer’s research, as exemplified by the achievements of scientists like Beth Stevens. With substantial financial support from the National Institutes of Health and other agencies, researchers can explore uncharted territories that promise to unlock new treatment paths and enhance our understanding of the disease. These funds enable long-term studies crucial for tracking the disease’s evolution and the effects of potential treatments on microglial functioning and neuronal health.
As the fight against Alzheimer’s intensifies, continued investment in basic science is essential to uncover transformative insights. Stevens’ work on microglial cells demonstrates how foundational research can lead to significant breakthroughs in neurodegenerative disease understanding and treatment. The synergy between federal funding and scientific exploration is vital for fostering innovation and devising comprehensive strategies to combat Alzheimer’s and improve the lives of millions affected by this debilitating disease.
Future Directions in Alzheimer’s Research
Looking ahead, the trajectory of Alzheimer’s research appears promising, particularly as new technologies and methodologies emerge to study neurodegenerative diseases. Scientists are increasingly utilizing advanced imaging techniques and genetic analyses to probe the complexities of the brain’s immune system, focusing on microglial cells. Beth Stevens’ innovative approach exemplifies the potential trapped within this research landscape, as shifting the focus towards the immune response offers new avenues for understanding and combatting Alzheimer’s. This evolution underscores the significance of interdisciplinary collaboration in driving the quest for effective treatments.
Moreover, integrating findings from various fields such as neuroimmunology and genomics will be key in shaping future research initiatives. The interactions between microglial behavior, synaptic health, and Alzheimer’s pathology present a complex puzzle that researchers are eager to solve. As collaborations expand and more resources become available, the future of Alzheimer’s research is poised to lead to exciting discoveries that will inform both clinical practices and innovative therapeutic developments aimed at preventing or eradicating the disease.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they function as the brain’s immune system. They patrol for illness or injury, clear dead or damaged cells, and prune synapses in the brain. However, abnormalities in this pruning process have been linked to neurodegenerative diseases like Alzheimer’s, making them a key focus for potential treatments and early detection biomarkers.
How has Beth Stevens contributed to the understanding of Alzheimer’s disease?
Beth Stevens has significantly advanced Alzheimer’s research by transforming our understanding of microglial cells and their role in the brain’s immune response. Her investigations revealed how abnormal synaptic pruning by these cells can lead to neurodegenerative diseases, including Alzheimer’s, paving the way for new treatment strategies and early diagnostic tools.
Why are microglial cells important for understanding neurodegenerative diseases like Alzheimer’s?
Microglial cells are vital for understanding neurodegenerative diseases like Alzheimer’s because they help maintain brain health by clearing cellular debris and regulating synapse function. Research shows that when microglial function is impaired, as seen in Alzheimer’s, it can contribute to disease progression, highlighting their importance in developing effective therapies.
What new treatments are being developed based on recent Alzheimer’s research?
Recent Alzheimer’s research, particularly that of Beth Stevens, is leading to potential new treatments that target microglial cell function. By understanding how these cells interact with neurons and their role in synaptic pruning, scientists aim to develop therapies that can correct these processes, ultimately improving outcomes for patients with Alzheimer’s and other neurodegenerative diseases.
How might advances in microglial cell research impact future Alzheimer’s treatment?
Advances in microglial cell research may dramatically impact future Alzheimer’s treatment by providing insights into the mechanisms of neuronal health and disease. As researchers uncover how microglial cells contribute to neurodegenerative processes, it could lead to the development of drugs that enhance their protective functions, mitigating the symptoms and progression of Alzheimer’s disease.
What are the potential biomarkers being identified in Alzheimer’s research?
Potential biomarkers being identified in Alzheimer’s research include indicators of microglial activity and synaptic health. By understanding the changes in these immune cells and their role in the brain’s immune system, researchers aim to develop early diagnostic tools that can detect Alzheimer’s before significant cognitive decline occurs.
| Key Areas | Details |
|---|---|
| Researcher: Beth Stevens | Neuroscientist at Boston Children’s Hospital and the Broad Institute. |
| Focus of Research | Transforming the understanding of microglial cells as the brain’s immune system. |
| Microglial Function | Patrol the brain for illness, clear damaged cells, and prune synapses. |
| Impact of Abnormal Pruning | Contributes to Alzheimer’s, Huntington’s, and other neurodegenerative disorders. |
| Funding Sources | Research primarily funded by NIH and federal grants. |
| Potential Impact | New medicines and biomarkers for early detection of Alzheimer’s. |
| Future Projections | Annual Alzheimer’s cases expected to double by 2050. |
Summary
Alzheimer’s research is at a pivotal juncture, particularly with the promising insights from neuroscientist Beth Stevens. Her groundbreaking work on microglial cells has revealed their crucial role in maintaining brain health and highlighted how their malfunction can lead to devastating conditions, including Alzheimer’s disease. As the prevalence of Alzheimer’s continues to rise dramatically, Stevens’ findings provide hope for innovative treatments and biomarkers that can significantly alter the course of this disease, potentially improving the lives of millions.