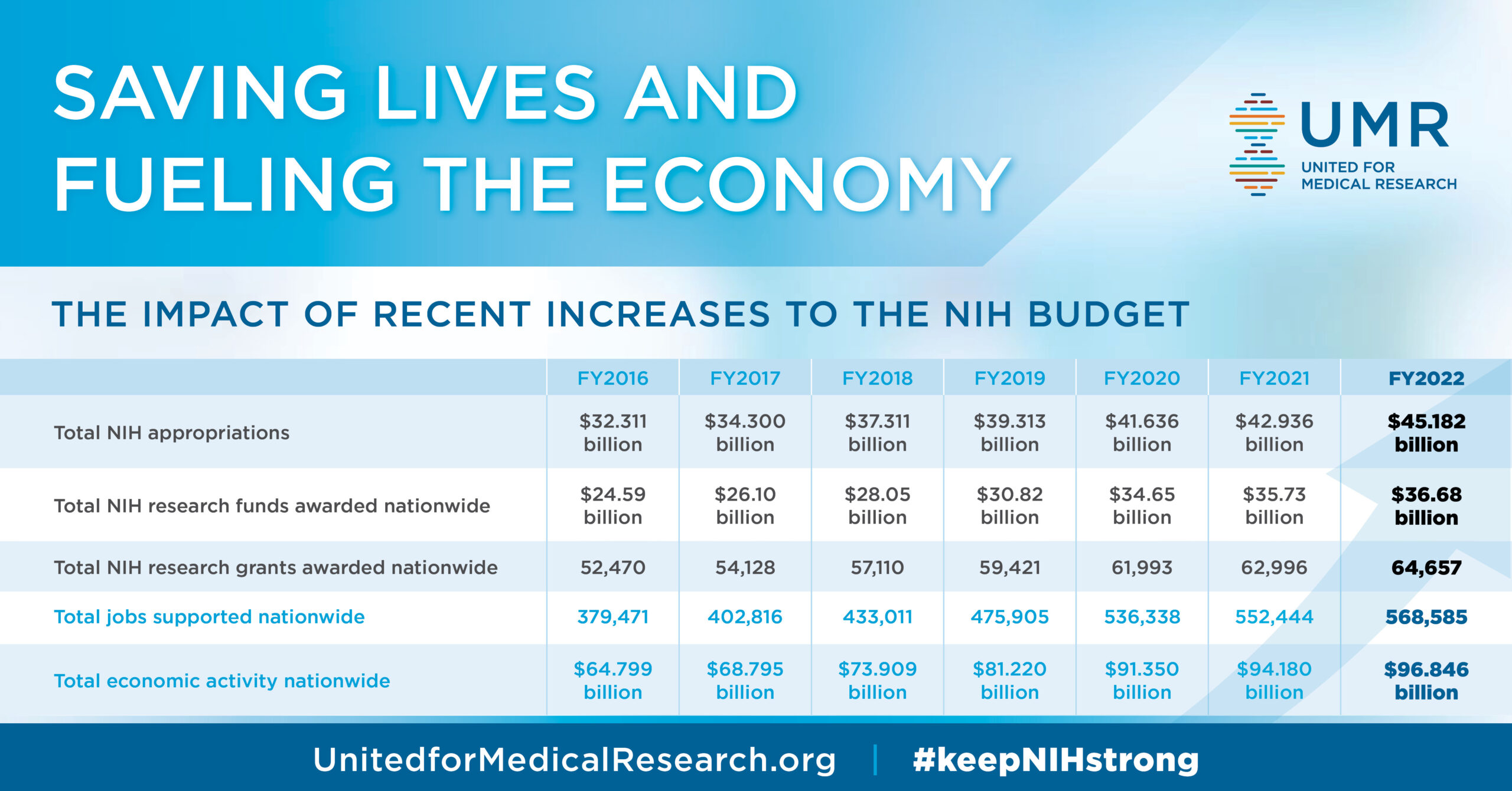
The impact of funding cuts on medical research is profound and far-reaching, threatening not only scientific advancements but also the very safety of patients enrolled in clinical trials. As federal research grants dwindle, crucial oversight mechanisms, governed by Institutional Review Boards (IRBs), face severe disruptions. This has raised significant concerns over patient safety in research, especially when compliance with ethical standards in medical research is jeopardized. Institutions reliant on medical research funding like the NIH are now struggling to uphold their commitments to safeguard human subjects amid these budget shortfalls. The ripple effect of such funding limitations could hinder groundbreaking discoveries and undermine public trust in the research process, making it essential to address these challenges urgently.
Funding reductions within the realm of health science pose critical challenges to research endeavors aimed at enhancing patient outcomes. With diminished financial resources, the integrity of research oversight, largely through IRB governance, becomes increasingly tenuous. The ramifications of this trend extend beyond academia, impacting community trust and ethical considerations in clinical trials. The National Institutes of Health (NIH) and other funding bodies are pivotal in maintaining the standards of patient protection that are foundational to ethical research practices. As we navigate this troubling landscape, the urgency to advocate for sustained investment in medical research becomes clear, ensuring the safety and welfare of research participants remain a top priority.
The Consequences of Funding Cuts on Medical Research Integrity
Funding cuts in medical research profoundly impact the integrity of studies designed to ensure patient safety. When financial resources are limited, institutions are often forced to reduce the number of studies they can conduct, leading to insufficient oversight. This neglect in funding compromises the ethical standards that underpin medical research, raising significant concerns about the safety and welfare of research participants. Moreover, the loss of financial support hampers the ability of Institutional Review Boards (IRBs) to conduct thorough evaluations of research proposals, resulting in potentially harmful research practices going unchecked.
Additionally, diminished funding affects the collaborative nature of modern research, as evident in the recent halt of federal grants, particularly to entities like Harvard. Researchers may be unable to divert needed resources towards the rigorous oversight that minimizes risks to human subjects. The scenario highlights an urgent need for sustained funding to guarantee that ethical standards are upheld and that research institutions can continue to foster a culture of accountability essential for patient safety.
Importance of NIH Funding in Safeguarding Patient Welfare
The National Institutes of Health (NIH) plays a pivotal role in protecting the interests of patients participating in research through its funding mechanisms. By providing financial support for studies that include stringent IRB oversight, the NIH helps ensure compliance with ethical standards that prioritize patient safety. Research funded by the NIH is continuously monitored not only for scientific advancement but also for ethical considerations, ensuring that the rights and welfare of participants are preserved. Moreover, this oversight is especially critical in clinical trials involving new drugs or treatments, where the potential for adverse effects is significant.
Furthermore, NIH funding assists in fostering a unified approach to research that spans multiple institutions and studies. The adoption of a single IRB (sIRB) model for multisite studies has been instrumental in streamlining ethical reviews while reinforcing the importance of patient safety. This collaborative model, backed by substantial NIH funding, enables institutions to share resources and ensure comprehensive oversight of participant protection, therefore enhancing the overall quality of medical research.
Significance of Institutional Review Boards (IRBs) in Research
Institutional Review Boards (IRBs) serve as the essential gatekeepers in medical research, tasked with reviewing and approving research proposals to protect the rights and safety of participants. The functions of IRBs include evaluating research methodology, obtaining informed consent, and ensuring that potential risks are thoroughly assessed and mitigated. This oversight is a critical component of ethical standards in medical research, making it essential that IRBs receive adequate support and funding to fulfill their responsibilities effectively. Without sufficient financial backing, IRBs may struggle to maintain rigorous oversight, leading to a compromised research environment.
The historical context of IRBs underscores their importance and the need for continued support in medical research. Past abuses highlighted the necessity for strict ethical oversight and established a framework that prioritizes participant safety. As funding cuts threaten to erode these protections, the potential risks to study participants become increasingly significant, demonstrating the crucial role that adequate funding plays in the preservation of both ethical and scientific integrity within research.
How Funding Cuts Impede Research Progress and Patient Safety
The adverse effects of funding cuts on medical research cannot be overstated, as they directly impede progress and have dire implications for patient safety. Interruptions in research activities often halt clinical trials mid-process, preventing essential data from being gathered and analyzed. This not only prolongs the timeline for crucial therapies to reach the market but also leaves participants in limbo, potentially exposing them to ongoing risks without the oversight they require. Such disruptions can lead to a loss of trust in the medical research field, making individuals less likely to participate in future studies.
Cuts to research funding also limit the capacity of institutions to implement the necessary regulatory compliance and safety net required to protect research participants. This lack of resources can result in insufficient monitoring of adverse events and ineffective communication regarding patient risks and benefits. Therefore, the need for adequate funding is paramount to safeguard the integrity of medical research and protect the health and safety of the public.
Historical Developments Leading to Funding Support for Patient Safety
The evolution of medical research funding has been influenced by historical events that reveal the importance of ethical oversight and patient safety. Events such as the Tuskegee Study and the Nuremberg Trials emphasized the need for stringent ethical standards and the establishment of independent oversight bodies like IRBs. These historical injustices have shaped public perception and policy around medical research, showcasing the necessity for continuous funding to uphold ethical standards in research. Moreover, the establishment of frameworks and regulatory bodies reflecting ethical oversight has increased the demand for research funding to ensure compliance with safety protocols.
The recognition of past medical experimentation failures has propelled funding initiatives aimed at enhancing the transparency and oversight of health research. As funding from organizations such as the NIH emphasizes ethical considerations in medical research, it becomes more vital to secure financial support for IRB operations that facilitate effective oversight. This funding allows for the cultivation of a research environment where participants can trust that their rights and safety will not be compromised.
Current Challenges Facing Medical Research Funding
Current challenges in medical research funding are multi-faceted, with the recent freeze of federal grants posing immediate risks to patient safety and research integrity. The abrupt stop to financial support hampers the ability to conduct essential oversight that ensures compliance with ethical standards. Consequently, ongoing studies must grapple with interruptions that could compromise the safety of participants involved in medical trials. Such challenges reflect the broader systemic issues within funding mechanisms that prioritize immediate financial constraints over long-term impacts on patient health and safety.
Moreover, the competitive landscape for available research funding creates additional pressure on institutions to secure grants, often diverting focus from ethical practices to financial survival. Establishing a reliable and consistent funding stream is crucial to maintaining a robust framework for ethical oversight, as it enables research entities to invest in the necessary infrastructure and training that supports patient safety. Without addressing these funding challenges, the integrity of medical research and the safety of participants are placed at significant risk.
The Role of Collaborative Research in Enhancing Patient Safety
Collaborative research is increasingly recognized as a vital approach to enhance patient safety in medical studies. By pooling resources, expertise, and funding across institutions, research teams can establish comprehensive oversight mechanisms that uphold ethical standards. This collaboration is particularly significant in multisite research, where shared IRB review practices facilitate a more streamlined and effective evaluation process. The positive outcomes of this collaborative approach subsequently reinforce the importance of adequate funding to support such partnerships.
In a collaborative model, researchers can monitor patient recruitment and data collection across various sites, leading to more rigorous safety protocols and a commitment to transparency. When financial support is adequate, institutions can invest in dedicated resources that focus on the protection of study participants, underscoring the notion that collaborative research not only advances science but actively safeguards public health. Thus, ongoing funding support is essential to ensuring that collaborative research remains a cornerstone in promoting patient safety.
Strategies for Securing Funding in Medical Research
To address the significant funding cuts threatening medical research, stakeholders must implement innovative strategies to secure financial resources. Building partnerships with government agencies, non-profits, and private sectors can create a diverse funding ecosystem that supports research integrity and patient safety. Engaging in advocacy efforts to highlight the necessity of research funding can also play a critical role in influencing policy changes that prioritize ethical oversight and support evolving research in the medical domain.
Moreover, embracing new funding models, such as crowdfunding or public-private collaborations, can help researchers gain financial support while enhancing community involvement in the research process. By fostering an inclusive approach that emphasizes transparency and patient engagement, researchers can build a robust advocacy network. These strategies can mitigate the effects of funding cuts while maintaining a strong commitment to ethical oversight and patient safety in medical research.
Future of Medical Research Funding: Ensuring Ethical Standards
The future of medical research funding must focus on ensuring ethical standards amid ongoing challenges and cuts. As the landscape of funding evolves, maintaining a rigorous commitment to patient safety must remain at the forefront of research initiatives. Institutions need to advocate for consistent, transparent funding sources that uphold the values of integrity and ethical oversight. Stakeholders must collectively work to emphasize the importance of sufficient funding not just for research advancement, but for safeguarding public trust and health.
In navigating future funding challenges, it will be essential for medical research institutions to innovate in their approaches to financing. By prioritizing ethical considerations and patient welfare, the research community can foster a more stable, secure funding environment. It is imperative that funding supports the foundational role of oversight bodies like IRBs, ensuring that the health and safety of all research participants remain protected.
Frequently Asked Questions
What is the impact of funding cuts on medical research funding and patient safety?
Funding cuts significantly hinder medical research funding, causing delays in crucial studies that protect patient safety. These cuts lead to the cessation of ongoing projects, limiting the ability of Institutional Review Boards (IRBs) to oversee research effectively, which poses risks to participants’ rights and welfare.
How do funding cuts affect IRB oversight in medical research?
Funding cuts can severely disrupt IRB oversight in medical research by affecting the resources available for review processes. Limited funding can result in fewer IRB staff members, longer review times, and inadequate monitoring of participant safety, compromising ethical standards in medical research.
In what ways do cuts in NIH research funding impact ethical standards in medical research?
Cuts in NIH research funding can compromise ethical standards in medical research by reducing the capacity for thorough oversight by IRBs, leading to potential lapses in compliance with ethical guidelines. Without adequate funding, the necessary resources for ensuring informed consent and participant safety may be jeopardized.
Why is medical research funding crucial for protecting patient safety in research?
Medical research funding is vital for protecting patient safety because it supports the infrastructure necessary for comprehensive IRB reviews. These funds enable institutions to evaluate research protocols rigorously, ensuring that patients are informed of risks and their rights are upheld during studies.
What are the long-term effects of funding cuts on medical research and patient outcomes?
Long-term effects of funding cuts on medical research can lead to stagnation in medical advancements, decreased innovation in patient care, and a deterioration of trust in the research community. As studies halt or slow down, the potential benefits for patient outcomes may be lost, affecting public health initiatives.
How do funding cuts disrupt collaborations in medical research institutions?
Funding cuts disrupt collaborations in medical research institutions by limiting resources available to coordinate research across multiple sites. Such constraints can hinder the implementation of overarching IRB oversight and prevent new partners from joining ongoing studies, delaying critical advancements in medical science.
What role do IRBs play in maintaining ethical standards amid funding cuts in medical research?
IRBs are essential for maintaining ethical standards in medical research, especially amidst funding cuts. They assess research proposals to ensure participant safety, monitor compliance with ethical guidelines, and address any concerns that arise, safeguarding the integrity of the research process and the welfare of participants.
What are the implications of halted research funding for patient safety in ongoing medical studies?
Halted research funding can have dire implications for patient safety in ongoing medical studies, as it may prevent continued oversight by IRBs and limit the ability to address any adverse events or safety concerns promptly, increasing risks to participants involved in the research.
| Key Points | Details |
|---|---|
| Funding Cuts Impact | $2 billion freeze in federal research grants affects patient safety and rights. |
| SMART IRB Role | A national system that oversees multi-site medical research to protect participant welfare. |
| Institutional Review Boards (IRBs) | IRBs ensure ethical standards through reviews of research proposals, promoting safety and informed consent. |
| Public Trust | Funding cuts could deepen skepticism and distrust in clinical research. |
| Consequences on Research | Halting studies risks participants and limits collaborative innovation. |
| Historical Context | Past unethical research highlights the necessity of oversight from IRBs. |
Summary
The impact of funding cuts on medical research is profound and troubling. The recent freeze of over $2 billion in federal research grants disrupts essential protections for patients engaged in clinical studies. This halt threatens the integrity of ongoing research efforts, jeopardizes public trust, and limits innovation in medical advancements. Institutional Review Boards (IRBs) play a critical role in ensuring ethical oversight, but without adequate funding, the ability to safeguard rights and welfare of research participants is severely compromised. Consequently, there is a pressing need to reinforce funding for medical research to maintain ethical standards and ensure the safety of patients.