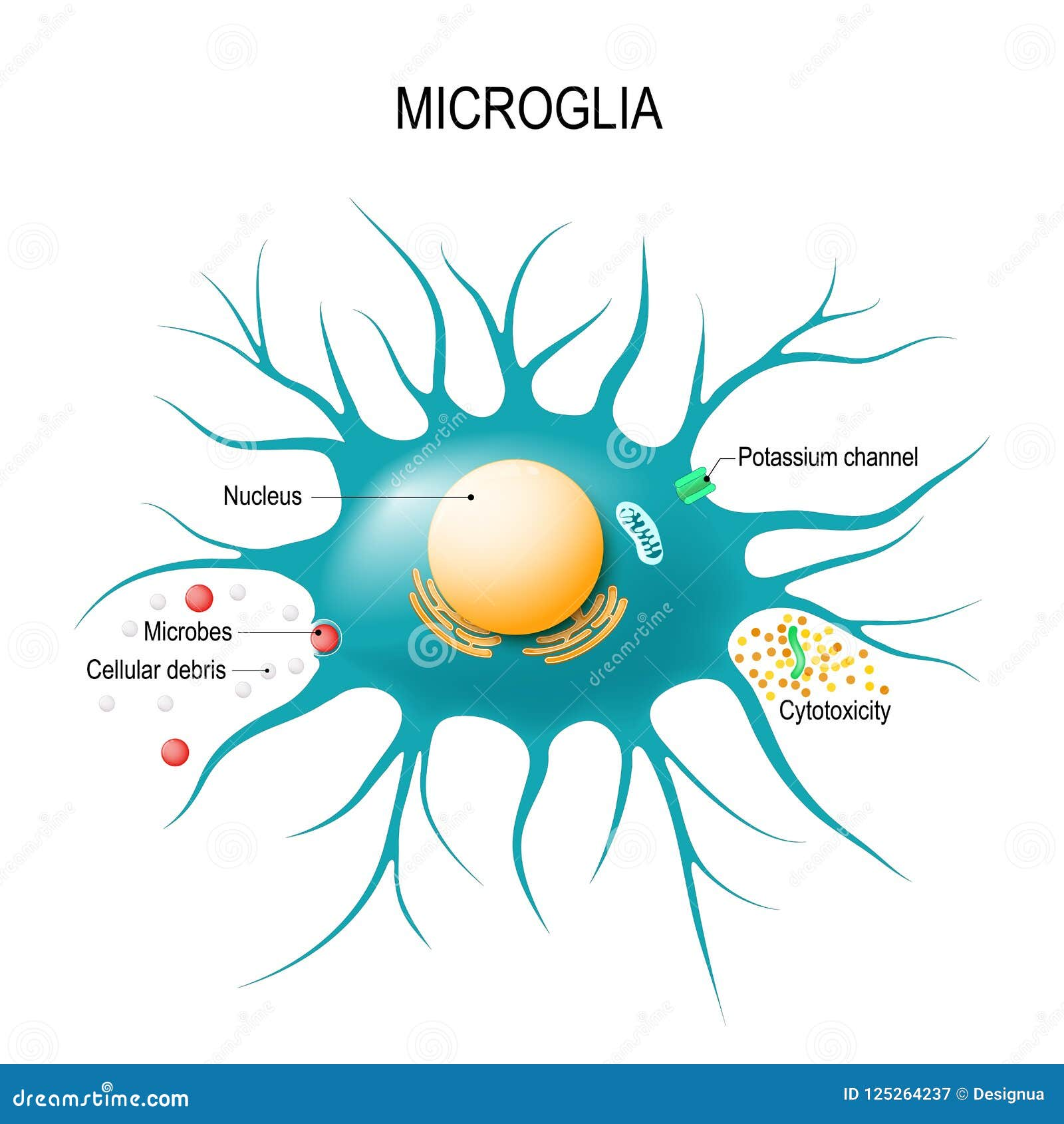
Microglial cells play a pivotal role as the brain’s immune defenders, safeguarding our neural environment while actively participating in vital processes like synaptic pruning. These specialized cells are not just passive observers but are dynamic participants in brain immunity, fiercely guarding against diseases such as Alzheimer’s. Research led by prominent neuroscientist Beth Stevens has unveiled how microglia’s missteps in cleaning up during neural development can lead to detrimental effects in neurodegenerative diseases. Given their significant involvement in both the progression and potential treatment of Alzheimer’s disease, understanding microglial behavior has become essential in neuroscience. As we delve deeper into their function, we uncover new pathways for therapeutic intervention, illuminating hope for millions affected by these conditions.
Also known as the brain’s resident immune cells, microglia are instrumental in maintaining neural health through their monitoring and maintenance roles. Often described as the custodians of our central nervous system, these cells engage in a process known as synaptic pruning, wherein they remove unnecessary connections between neurons, thereby optimizing brain function. Research from Dr. Beth Stevens has made significant contributions to understanding how dysfunction in these cells may lead to harmful neurodegenerative conditions, particularly Alzheimer’s disease. By investigating the intricacies of microglial activity, scientists are gaining insights into how these immune cells interact with neuronal pathways and contribute to the broader framework of brain health. As the fight against cognitive decline advances, redefining the role of these vital cells remains crucial in developing innovative therapies.
Understanding Microglial Cells and Their Role in Brain Immunity
Microglial cells are integral to the brain’s immune response, acting as vigilant sentinels that monitor the central nervous system for signs of damage or disease. As the brain’s innate immune cells, they are responsible for removing dead neurons and debris, a process crucial for maintaining healthy brain function. In the context of neurodegenerative diseases like Alzheimer’s, the role of microglia becomes even more pronounced. Research has shown that these cells can shift from benevolent guardians to harmful entities, particularly when they engage in abnormal synaptic pruning at an accelerated rate.
Beth Stevens’ pioneering research has shed light on how microglial cells can contribute positively or negatively to brain health. Her studies revealed that while microglia are essential for neuroprotection and synaptic remodeling during normal development, dysregulation in their activity is linked to the progression of Alzheimer’s disease and other neurodegenerative disorders. By unraveling the complexities of microglial functions, Stevens not only emphasizes their importance in brain immunity but also opens avenues for novel therapeutic strategies aimed at rebalancing microglial activity to prevent or mitigate the effects of diseases like Alzheimer’s.
The Impact of Synaptic Pruning on Neurodegenerative Diseases
Synaptic pruning is a crucial developmental process where unnecessary neuronal connections are eliminated to optimize brain circuitry. While this process is vital during early brain development, abnormalities in synaptic pruning have been implicated in several neurodegenerative diseases, including Alzheimer’s. In the pursuit of understanding this phenomenon, researchers like Beth Stevens have demonstrated that microglial cells play a key role in regulating synaptic pruning. Excessive or inappropriate pruning can lead to the loss of critical neuronal connections, resulting in cognitive decline and other symptoms associated with Alzheimer’s.
Stevens’ research suggests that targeting the mechanisms behind synaptic pruning could lead to innovative treatment strategies for Alzheimer’s disease. By understanding how microglial cells contribute to synaptic dynamics, scientists may develop interventions that can correct or halt the detrimental pruning processes. This insight not only paves the way for potential biomarkers but also informs drug development geared towards preserving neuronal integrity and function in patients afflicted by neurodegenerative diseases.
Furthermore, by investigating the effects of abnormal microglial activation on synaptic pruning, Stevens highlights the potential of using immunomodulatory therapies to alter microglial behavior, thereby restoring normal synaptic pruning processes. This could ultimately translate into more effective interventions for slowing the progression of Alzheimer’s disease and improving patient outcomes.
Innovations in Alzheimer’s Research Through Curiosity-Driven Science
The journey of discovery in Alzheimer’s research has been significantly propelled by curiosity-driven science, exemplified by Beth Stevens’ commitment to exploring the unknown facets of brain function. By focusing on microglial cells and their impact on synaptic pruning, Stevens has not only made strides in understanding the underlying mechanisms of Alzheimer’s disease but has also spotlighted the importance of foundational research. Her work serves as a testament to the notion that basic science often lays the groundwork for practical applications, leading to significant advancements in our understanding of neurodegenerative diseases.
Stevens’ recognition as a MacArthur ‘genius’ reflects the impact of her innovative approach to studying microglia in both healthy and diseased states. With sustained funding from the NIH and other sources, her lab continues to explore the nuanced interactions between microglial activity and neural circuitry. This focus on curiosity-driven research highlights the interconnectedness of basic neuroscience and clinical applications, reinforcing the idea that understanding the intricacies of brain immunity can yield breakthroughs in the treatment of Alzheimer’s disease.
The Future of Alzheimer’s Disease Treatment: A Molecular Perspective
As researchers like Beth Stevens delve deeper into the molecular underpinnings of Alzheimer’s disease, an exciting future emerges for treatment strategies targeting microglial cells and their role in synaptic pruning. The notion that these immune cells can both protect and endanger neuronal health leads to innovative approaches aimed at harnessing their beneficial properties while mitigating harmful effects. This duality in microglial function presents an opportunity for developing novel pharmacological agents that can regulate microglial activity and restore homeostasis in the brain.
The potential application of such therapies could revolutionize the landscape of Alzheimer’s treatment. By effectively targeting the pathways involved in microglial response and synaptic remodeling, researchers hope to develop drugs that prevent the premature death of neurons and the associated cognitive decline typically observed in Alzheimer’s patients. Continued collaboration between basic science and clinical research will be crucial to translating these insights into viable treatment options that not only slow disease progression but ultimately enhance the quality of life for millions affected by neurodegenerative diseases.
Beth Stevens’ Influence on Neuroscience and Alzheimer’s Research
Beth Stevens’ influence extends far beyond her groundbreaking discoveries; it resonates through the scientific community as she inspires future generations to explore the complexities of brain health. As an academic at Harvard Medical School and a leader at Boston Children’s Hospital, she emphasizes the critical need for research that bridges basic science with practical implications for diseases like Alzheimer’s. By fostering an environment where curiosity-driven investigations thrive, Stevens cultivates a culture of innovation that encourages researchers to tackle challenging questions surrounding the brain’s immune system and its role in neurodegenerative diseases.
Her emphasis on the importance of microglial cells in shaping our understanding of synaptic interactions has pivoted the focus of many research programs towards the cellular and molecular mechanisms involved in Alzheimer’s. With her research funded by prestigious institutions and her voice amplified through various forums, Stevens continues to advocate for increased awareness and support for Alzheimer’s research. Her contributions not only enrich our knowledge but also drive a collective effort to seek solutions for conditions that currently have no cure.
Advancing Alzheimer’s Biomarkers Through Microglial Research
In the relentless pursuit of effective diagnostics and treatments for Alzheimer’s disease, the study of microglial cells has provided crucial insights that may lead to the identification of novel biomarkers. Beth Stevens’ research highlights how abnormal microglial activity and synaptic pruning patterns can serve as indicators of disease progression. By uncovering these relationships, her work contributes to the broader goal of developing biomarkers that can detect Alzheimer’s in its early stages, potentially allowing for interventions before irreversible neuronal damage occurs.
The establishment of reliable biomarkers is essential for enhancing clinical trials and improving patient stratification in therapeutic research. As Stevens and her team continue to elucidate the complexities of microglial functions, they pave the way for future studies aimed at correlating specific microglial behaviors with clinical outcomes in Alzheimer’s patients. The potential to track disease progression through these biomarkers could significantly change the way we approach both diagnostics and treatment planning, ultimately leading to more personalized and effective strategies in managing Alzheimer’s disease.
The Connection Between Neuroinflammation and Alzheimer’s Disease
Neuroinflammation has become a key concept in understanding the pathophysiology of Alzheimer’s disease. Microglial cells, while essential for neuroprotection, can also contribute to a state of chronic inflammation when dysregulated. Research led by Beth Stevens has illustrated how alterations in microglial responses can lead to increased levels of neuroinflammation, which has been closely associated with the progression of Alzheimer’s and other neurodegenerative disorders. Understanding the fine line between beneficial and detrimental microglial activity is crucial for unraveling the complexities of neuroinflammation in the context of Alzheimer’s.
Addressing neuroinflammation presents another potential therapeutic target for Alzheimer’s disease. By modulating microglial responses, researchers can shift the balance towards a protective immune environment. Strategies aimed at reducing chronic neuroinflammation could not only help to slow the progression of Alzheimer’s but also improve cognitive function in affected individuals. Through ongoing research that intersects neurobiology and immunology, a clearer picture of the role microglial cells play in neuroinflammation and its impact on Alzheimer’s will emerge, providing new pathways for intervention.
The Role of Federal Funding in Alzheimer’s Research
Federal funding has been pivotal in advancing Alzheimer’s research, enabling scientists like Beth Stevens to pursue groundbreaking studies into the roles of microglial cells and neuroinflammation. Through grants from the National Institutes of Health (NIH), Stevens has conducted extensive research that has defined the relationships between microglial activity and synaptic dynamics in the context of Alzheimer’s disease. This sustained financial support is vital for fostering innovative studies that push the boundaries of existing knowledge and lead to impactful discoveries.
The importance of federal investment in health research cannot be understated, especially as the aging population continues to grow and the prevalence of Alzheimer’s disease escalates. By providing the resources needed for curiosity-driven exploration, federal agencies facilitate the emergence of new ideas and therapeutic approaches in Alzheimer’s research. As Stevens has showcased, successful research programs rely on a foundation of robust funding, which ultimately leads to advancements that can change the trajectory of care for millions living with neurodegenerative diseases.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells are crucial components of the brain’s immune system and are involved in monitoring brain health. In Alzheimer’s disease, they can become dysfunctional, leading to abnormal synaptic pruning and ineffective removal of dead cells, which contributes to neurodegeneration.
How do microglial cells affect neurodegenerative diseases like Alzheimer’s?
Research has shown that microglial cells can impact neurodegenerative diseases such as Alzheimer’s by altering the process of synaptic pruning. Abnormal pruning by microglia can lead to the loss of vital synaptic connections, exacerbating cognitive decline in conditions like Alzheimer’s.
What is synaptic pruning, and how is it related to microglial cells?
Synaptic pruning is the process by which microglial cells eliminate unnecessary synapses, ensuring neural circuits function optimally. In Alzheimer’s disease, this process can become dysregulated, resulting in excessive or insufficient pruning, which is detrimental to brain health.
What discoveries about microglial cells has Beth Stevens made in her research?
Beth Stevens has contributed significantly to understanding microglial cell function, particularly how they regulate synaptic pruning. Her research highlights the link between dysfunctional microglia and neurodegenerative diseases like Alzheimer’s and lays the groundwork for potential biomarkers and treatments.
Why are microglial cells essential for brain immunity?
Microglial cells serve as the brain’s primary immune system, defending against pathogens and removing debris from injured or dying neurons. Their ability to respond to brain injuries and changes is vital in maintaining neurological health and preventing diseases such as Alzheimer’s.
Can abnormal microglial activity lead to neurodegenerative diseases?
Yes, abnormal microglial activity can contribute to neurodegenerative diseases. For instance, in Alzheimer’s, microglia may mistakenly prune healthy synapses, leading to cognitive decline. Understanding these mechanisms is key to developing therapies.
How might research on microglial cells transform treatment for Alzheimer’s disease?
Research on microglial cells can lead to innovative treatments for Alzheimer’s disease by identifying new drug targets and biomarkers. By focusing on normalizing microglial function, scientists aim to restore proper synaptic pruning and brain health.
| Key Aspect | Description |
|---|---|
| Microglial Cells | Act as the immune system of the brain, monitoring and responding to illness or injury. |
| Role in Alzheimer’s Disease | Abnormal pruning of synapses by microglia can contribute to Alzheimer’s and other neurodegenerative diseases. |
| Beth Stevens | A leading researcher who studies the role of microglial cells in brain health and disease. |
| Importance of Basic Science | Foundational research has led to breakthroughs in understanding microglial functions and potential treatments for diseases. |
| Funding | Research supported primarily by NIH grants, illustrating the importance of federal funding in scientific discovery. |
Summary
Microglial cells are crucial to brain health, functioning as the brain’s immune system by identifying and responding to damage. Recent advancements in research, particularly by scientists like Beth Stevens, have unveiled the significant role these cells play not only in regular brain maintenance but also in neurodegenerative diseases such as Alzheimer’s. Understanding microglial cells and their behavior is essential for developing new therapies and improving the care of millions affected by these conditions.